I sometimes take antibiotics for granted. As a doctor, I have prescribed thousands of courses of antibiotics throughout my career. I have taken many courses myself also, and they almost certainly saved my life when I perforated my appendix in my mid-20s.
Antibiotics are our mightiest weapons against disease and are a significant factor in our ever-improving quality of life and increasing life spans. The times are changing though, and the threat of antibiotic resistance now hangs over our heads. The rapidly expanding number of bacterial infections that are developing antibiotic-resistant strains are frequently featured on the news, and there is concern that we stand on the precipice of an antibiotic apocalypse. Are we going to return to the pre-antibiotic era, and if so, what should we expect?
Life before antibiotics
Infection has been the scourge of humanity throughout its entire history. Before the advent of antibiotics, the simplest cut or wound could be fatal, and any form of surgery was very risky indeed. Mothers and children commonly died from postnatal infections following childbirth, and mortality rates are now approximately 50 times lower than they were in the 1920s.
In the 1800s tuberculosis caused almost 25% of all deaths. Improvements in sanitation and public health measures decreased this figure dramatically by the 1900s, but there was still no cure in the pre-antibiotic era. In fact, the only treatment for tuberculosis at that time was a trip to the countryside for some fresh air. Even sexually transmitted diseases, most of which are so easily treatable today, were deadly killers and often caused terrible disfigurement to the sufferers.
All this changed in 1928 though, with the groundbreaking work of Sir Alexander Fleming and that of Sir Howard Florey, Sir Ernst Boris Chain, and Norman Heatley after him.
Fleming’s discovery
In 1928 a bacteriologist by the name of Alexander Fleming was working in the inoculation department of St. Mary’s Hospital. He had been on a summer vacation in Scotland and returned to find a messy lab bench with a Staphylococcus culture plate that had been contaminated with a mould. Initially, he thought that this was just a spoiled experiment, but on closer inspection, he noticed that no bacteria was growing near to the mould. The mould had created a bacteria-free circle around itself.
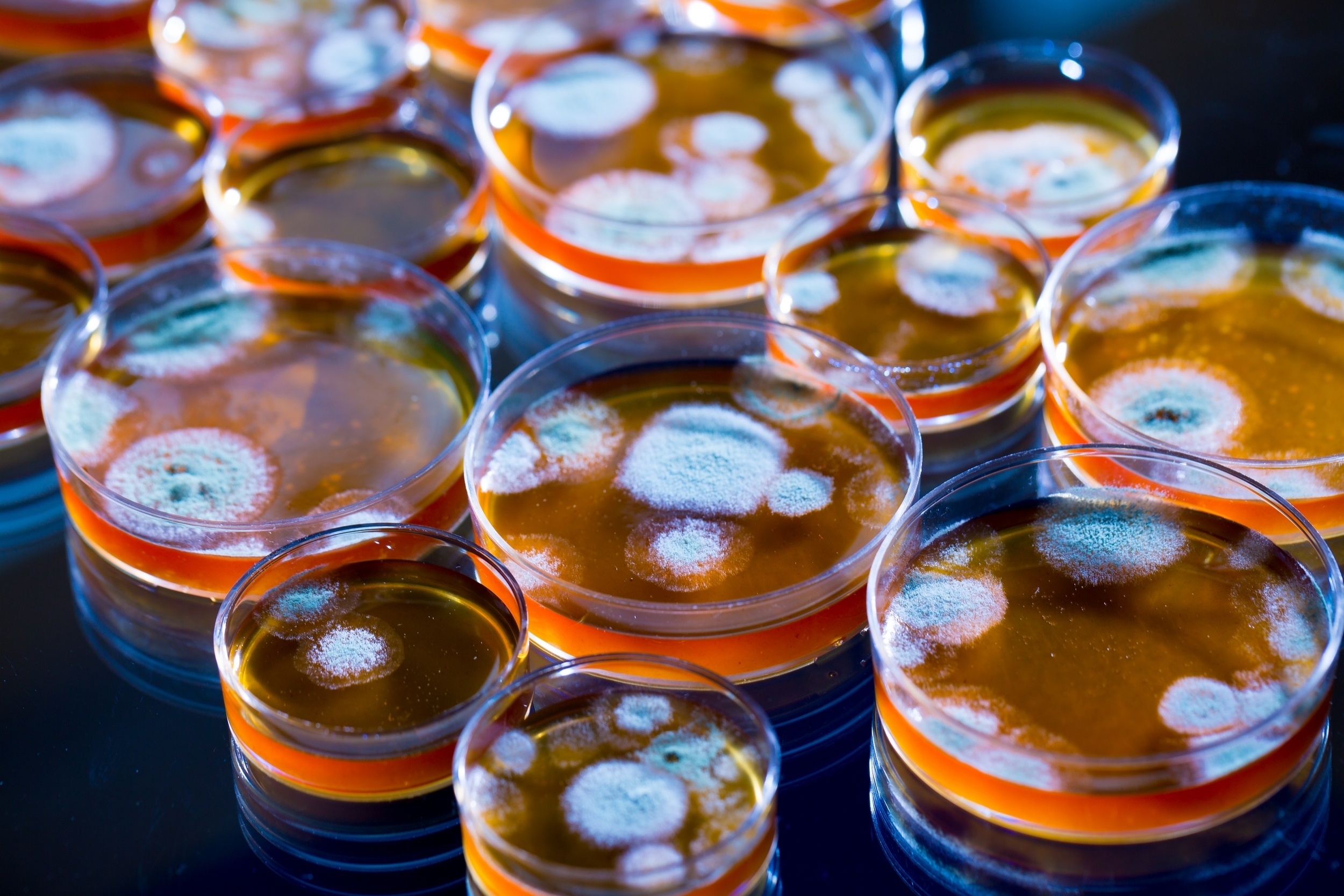
Penicillium spp. growing on a variety of Petri dishes (Image used on licence from Shutterstock)
Fleming performed further tests and discovered that other bacteria died on exposure to the mould, even some of the most dangerous pathogenic bacteria. The mould, known as Penicillium notatum, seemed to contain a factor that inhibited the growth of bacteria. He wrote later about his discovery:
“When I woke up just after dawn on September 28, 1928, I certainly didn’t plan to revolutionize all medicine by discovering the world’s first antibiotic, or bacteria killer. But I guess that was exactly what I did.”
Fleming went on to perform many more experiments using the mould and named the substance produced by it ‘penicillin’. He would never successfully extract purify the substance though, and in 1931 he ceased his work studying it.

Sir Alexander Fleming at his laboratory at St. Mary’s Hospital London
Chain and Florey
Fortunately, the story did not end there and two researchers at the University of Oxford, Sir Howard Florey and Sir Ernst Boris Chain, while planning a research project on natural bacterial killing substances, unearthed Fleming’s papers from 10 years earlier. Together the two scientists decided to investigate the antibacterial properties of the penicillin substance that Fleming had discovered.
They set to work on solving the chemical problems of extraction and purification that Fleming had been unable to overcome and found a way to produce a series of crude penicillin containing fluid extracts with the help of a young biochemist called Norman Heatley. In the summer of 1940, they designed an experiment in which a group of 50 mice were infected with the deadly Streptococcus bacteria. Half of the mice received penicillin, and half were left to battle the infection alone. Those that were given the penicillin lived and were healthy, while all of those that received the Streptococcus alone died from the infection.
As promising as the results of this experiment were the penicillin always lost potency when attempts were made to purify enough to make it suitable for use in humans. The first patient to be tested with penicillin was a 48-year-old Oxford police constable in September 1940 called Albert Alexander. He had suffered a scratch on his face from a rose bush, and it had become infected. Alexander became overwhelmed by sepsis and wasn’t responding to the sulfa drugs commonly used at the time. Upon hearing about the case, Florey and Chain asked the doctors treating Alexander if they could try penicillin. After five days of injections, Alexander began to recover, but unfortunately, the penicillin supplies ran out before the infection could be completely eradicated, and without the life saving antibiotic Alexander’s condition deteriorated again and he sadly died.
The Secret Weapon of World War II
It was now known that penicillin could cure bacterial infections, but the major hurdle was in finding a way to produce enough. At this point, it took over 2,000 litres of mould culture to obtain enough penicillin to treat a single case of sepsis in one person.
At this time World War II was raging, and there was an enormous need for antibiotics for both soldiers and civilians. Chain and Florey realised that penicillin could have an effect on the outcome of the war and searched for a solution to the problem of mass production. Florey would travel to the U.S.A with Heatley with samples of penicillin and would petition large pharmaceutical companies for their assistance.
In 1943, a bacteriologist working at a laboratory in Peoria, Illinois discovered a golden mould growing on a cantaloupe melon. This was a different mould from the Penicillium family called Penicillium Chrysogenum. A series of experiments were carried out on the mould, and it was discovered that if it was exposed to X-rays, a mutant strain was generated that could produce 1000 times more penicillin than Penicillium notatum.
The final piece of the puzzle was in place and mass production of penicillin was finally possible. By the end of 1943, hundreds of million units of pure penicillin were produced on a monthly basis. The impact on the war was huge. For the first time in history, the major killer in a war was not infection. By the end of the war, mass production was occurring in several labs in both the U.S.A and the U.K., and penicillin was now available around the world and was saving lives everywhere.

A technician preparing penicillin in 1943
The future
In an interview with The New York Times in 1945, Alexander Fleming warned that misuse of penicillin could result in the development of bacteria that were resistant to antibiotics. As he predicted the first resistant strains would appear by the mid-1950s. Over time new types of antibiotics would be produced, and an increasing number of infections became treatable, but no new antibiotics have been discovered since the 1980s. It seems increasingly likely that more and more of us will be affected by resistant strains of bacteria as time moves forward.
Doctors are now under huge pressure not to overprescribe antibiotics and worsen the situation, and attitudes are changing. We can all help the battle against resistance too, by following doctor’s advice and finishing the courses of antibiotics we are prescribed and not asking for antibiotics when we have a cold or the flu.
All hope is not lost though, and mankind is both ingenious and resourceful. Perhaps a new, as yet undiscovered antibiotic, will be found soon. The answer may even lie in the discovery of different antibiotic compounds, and promising research is underway looking at insect-based antibacterial substances and nanotechnology.





Recent Comments